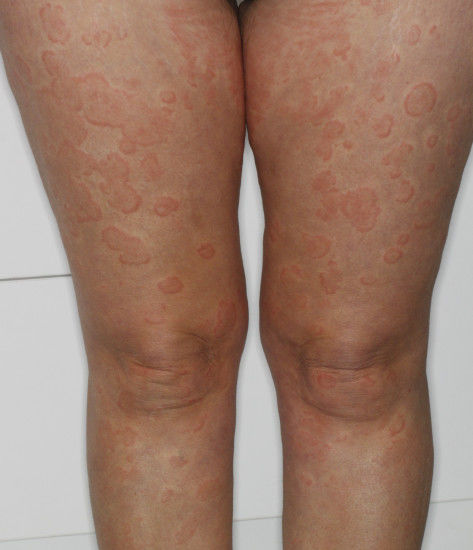
Those who suffer from chronic urticaria know how itchy this condition is. Also known as hives, the most common cause of chronic urticaria in adults is unknown. This can be a frustrating condition for patients.
What is Omalizumab?
Omalizumab (Xolair®) is a monoclonal antibody directed against IgE. It was first TGA (Therapeutic Goods Australia) registered on 13 June 2002 for the management of adults and adolescent patients with moderate allergic asthma. Recently, it has become approved for treatment of chronic urticaria.
This medication is only subsidised for severe urticaria and there are criteria which need to be fulfilled before this medication can be used.
The PBS (Pharmaceutical Benefit Scheme) criteria are:
- Severe urticaria without any known cause after investigation
- Severe urticaria that persists on a daily basis for at least 6 weeks in spite of H1 antihistamine treatment.
- Failed treatment for at least 2 weeks of:
- H1 antihistamine
- H2 antihistamine
- LTRA
- Doxepin
- High itch and urticaria score
How is Omalizumab administered?
Omalizumab is administered as a subcutaneous injection of 150 or 300mg every 4 weeks.
What are the side effects?
Omalizumab is generally well tolerated and side effects are rare. These include anaphylaxis and local injection site skin reactions.